ELAPRASE was shown to improve walking ability in Hunter syndrome patients aged 5 years and older.
The clinical trial evaluating ELAPRASE in patients aged 16 months to 5 years did not measure walking ability. However, treatment with ELAPRASE was shown to reduce spleen size in patients aged 16 months to 5 years, which was similar to the spleen size reduction shown with ELAPRASE in the clinical trial of patients 5 years and older.
A clinical trial of ELAPRASE in children under 16 months old has not been carried out; therefore, it is not established if ELAPRASE is safe and effective in children under 16 months old.
ELAPRASE mechanism of action
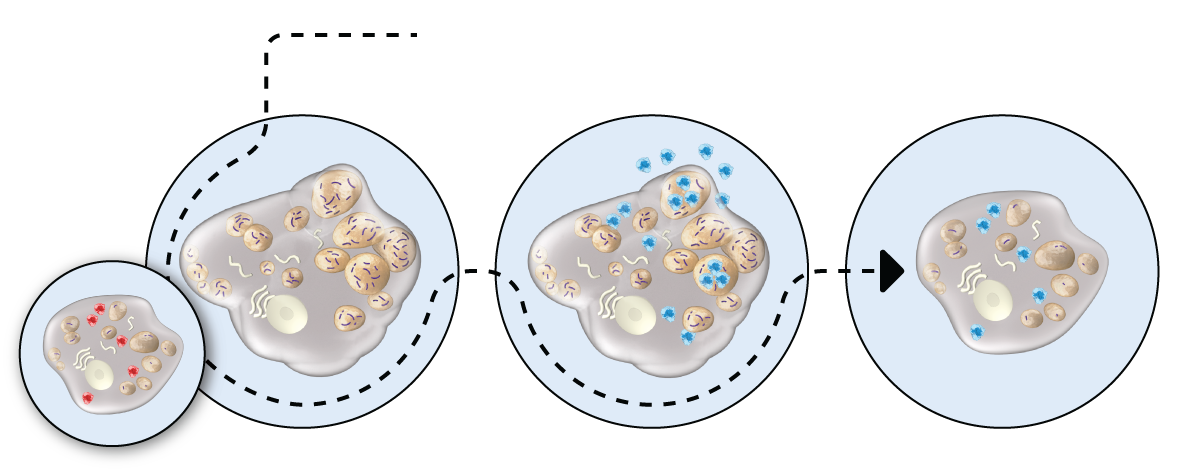
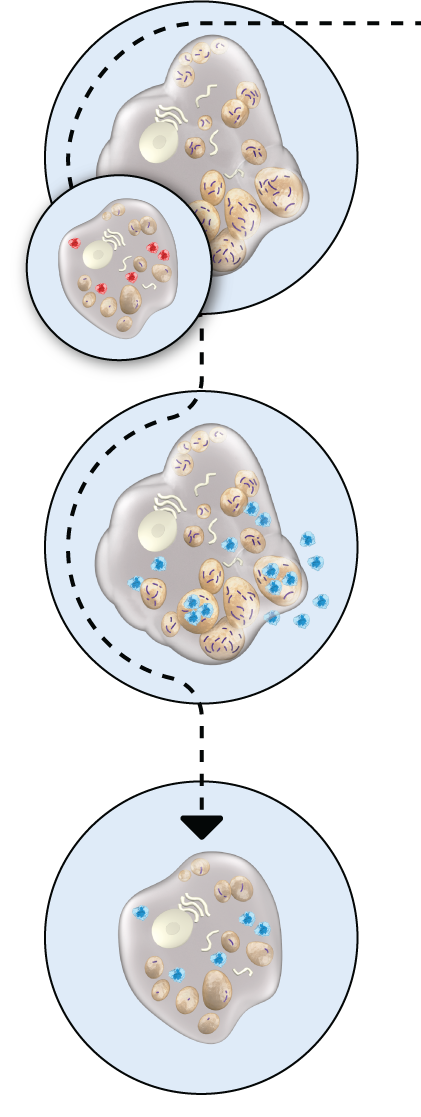
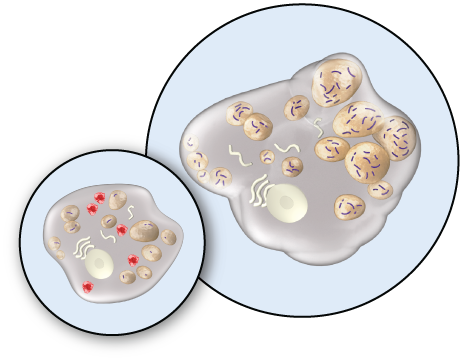
HEALTHY CELL VS. HUNTER SYNDROME-AFFECTED CELL
Hunter syndrome patients have insufficient iduronate-2-sulfatase enzyme activity. This means substances called glycosaminoglycans (GAG) build up in cells, causing cellular and organ damage.
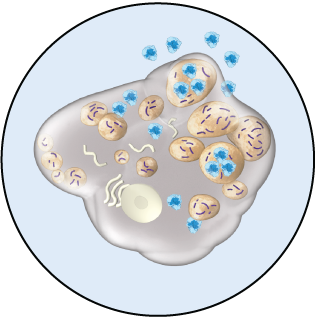
HOW ELAPRASE
WORKS
ELAPRASE is an enzyme replacement therapy. It is absorbed into cells where it breaks down GAGs, like the naturally occurring enzyme.
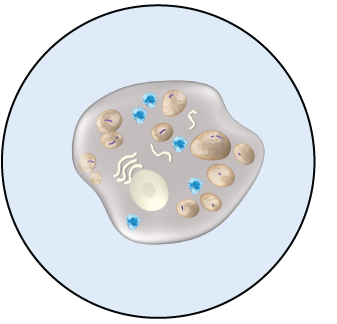
ELAPRASE-TREATED
CELL
ELAPRASE is intended to reduce the levels of GAGs in tissues. Decreases in urinary GAG levels are observed following treatment with ELAPRASE. The responsiveness of urinary GAG levels to dosage alterations of ELAPRASE is unknown, and the relationship of urinary GAG levels to other measures of clinical response has not been established.
Illustration only. Not intended to imply clinical significance.
RISK OF SERIOUS ALLERGIC REACTIONS
Some patients have experienced serious allergic reactions (including life-threatening anaphylactic reactions) during and up to 24 hours after treatment, regardless of how long they were taking ELAPRASE. Anaphylactic reactions are immediate and include breathing problems, low oxygen levels, low blood pressure, hives and/or swelling of the throat or tongue. If a patient (you or your child) has experienced an anaphylactic reaction, the patient may require an extended period of observation by the patient’s healthcare team. If you or your child has breathing problems, a fever, or a respiratory illness, you or your child may be at risk of life-threatening worsening of those conditions due to allergic reactions from ELAPRASE. Your healthcare team should be advised of those conditions before treatment with ELAPRASE because the information may affect the timing of ELAPRASE treatment.