Clinical trials are a scientific way to measure and compare the effectiveness and safety of a treatment with something else, to determine if a treatment can help patients. The data can be analyzed by statistical tests to check that the observation is not random chance.
Clinical trials in patients 5 years and older
The safety and efficacy of ELAPRASE were evaluated in a 12-month clinical study of 96 patients with Hunter syndrome aged 5 years and older. The trial was a double-blind, placebo-controlled study, meaning that it compared the effects of using ELAPRASE in some patients with using no ELAPRASE in other patients, without the doctors knowing which patient was treated with what (so that the measurements could not be biased by this information).
This study looked at the possibility of two different ELAPRASE dosing schedules, comparing them with placebo. These data were used to support the approved dosing of the 0.5 mg per kg of body weight once per week infusion schedule.

Hypersensitivity reactions were reported in 69% (22 of 32) of patients in the ELAPRASE once-weekly group.
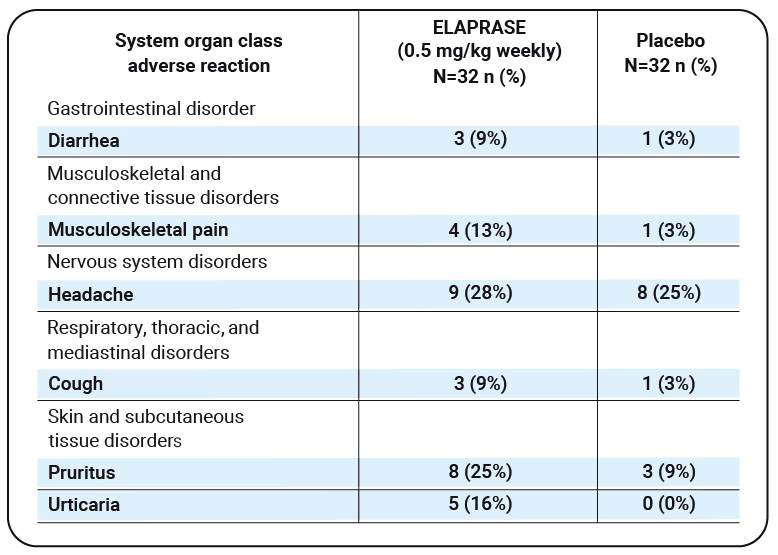
Adverse reactions that occurred in at least three patients (≥9%) in the ELAPRASE once-weekly group and had a higher incidence than in the placebo group are shown below:
Table 1
| System organ class adverse reaction |
ELAPRASE (0.5 mg/kg weekly) N=32 n (%) |
Placebo N=32 n (%) |
|---|---|---|
| Gastrointestinal disorder | ||
| Diarrhea | 3 (9%) | 1 (3%) |
| Musculoskeletal and connective tissue disorders |
||
| Musculoskeletal pain | 4 (13%) | 1 (3%) |
| Nervous system disorders | ||
| Headache | 9 (28%) | 8 (25%) |
| Respiratory, thoracic, and mediastinal disorders |
||
| Cough | 3 (9%) | 1 (3%) |
| Skin and subcutaneous tissue disorders |
||
| Pruritus | 8 (25%) | 3 (9%) |
| Urticaria | 5 (16%) | 0 (0%) |
Additional adverse reactions that occurred in at least 9% of patients (≥3 patients) in the ELAPRASE 0.5 mg/kg once every other week group, and occurred more frequently than in the placebo group, included rash (19%), flushing (16%), fatigue (13%), rapid heart rate (9%), and chills (9%).
Please see the ELAPRASE Important Safety Information here.
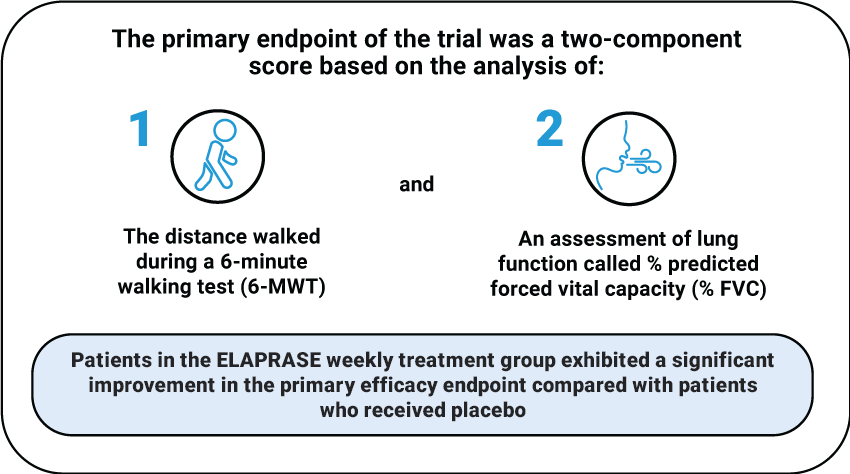
The primary endpoint of the trial was a two-component
score based on the analysis of:
1

The distance walked
during a 6-minute
walking test (6-MWT)
and
2

An assessment of lung
function called % predicted
forced vital capacity (% FVC)
Patients in the ELAPRASE weekly treatment group exhibited a significant improvement in the primary efficacy endpoint compared with patients who received placebo
When the individual components were examined separately, patients receiving ELAPRASE treatment for 12 months could walk an average 35 meters more in 6 minutes than patients receiving placebo—this difference was statistically significant. The difference in % predicted FVC was not statistically significant.
The clinical trial also measured levels of GAGs in patients' urine and the size of their livers and spleens to assess whether GAG storage levels inside the body were improved by ELAPRASE. Results showed that the placebo did not reduce liver and spleen size, whereas ELAPRASE once weekly reduced liver and spleen size. Additionally, the placebo did not alter urine GAG levels; ELAPRASE once weekly reduced urine GAG levels, although in half of the patients, the urine GAG levels were still considered to be higher than normal.
Clinical trial in patients 7 years and younger
A clinical trial evaluating the safety of ELAPRASE in 28 patients aged 16 months to 7 years was carried out, but did not measure walking ability as it was impractical to do so due to age group based on disease state. However, treatment with ELAPRASE was shown to reduce spleen size in patients aged 16 months to 5 years, which was similar to the spleen size reduction shown with ELAPRASE in the clinical trial of patients 5 years and older.
The safety results of this study showed that patients with certain types of genetic mutations experienced a higher number of allergic reactions, serious side effects, and development of an immune response to treatment. This immune response may interfere with the effectiveness of ELAPRASE. Talk to your healthcare team about whether you or your child may be at risk.
Patients experienced similar adverse reactions as those observed in clinical trials in patients 5 years and older, with the most common adverse reactions following ELAPRASE treatment being allergic (hypersensitivity) reactions (57%). Higher incidences of the following common hypersensitivity reactions were reported in this younger age group: fever (36%), rash (32%), and vomiting (14%). The most common serious adverse reactions occurring in at least 10% of patients (≥3 patients) included: bronchopneumonia/pneumonia (18%), ear infection (11%), and fever (11%).